24-Hour Urine Versus Dried Urine Hormone Testing
Introduction
24-hour urine hormone testing has long been established as a reliable method for evaluating the production of steroid hormones and their metabolites. In clinical practice, these levels correlate well with symptoms as well as with doses of exogenous hormones and symptom control. (1-3) Serum hormones are limited in their utility by comprising only a “snapshot” of the moment of collection and because, with the exception of testosterone and cortisol, only bound hormones are measured. Bound hormone levels may not be representative of true bio-available (free and conjugated) circulating hormones. Saliva does measure free, rather than bound, hormone but shares with serum the limitation of capturing only a single point in time. The exception to this is the popular four-point cortisol assay, which employs four collection points to define a circadian cortisol curve. Neither serum nor saliva allows for the measurement of hormone metabolites, and this is where the depth and breadth of information available from urine testing become apparent. Measuring hormone metabolites can provide the practitioner with invaluable insight into how a woman metabolizes her estrogens, with implications for both cancer risk and osteoporosis. It also allows comparison of Phase I and Phase II estrogen metabolites, giving a peek into the effectiveness of a patient’s methylation pathways. Similar advantages of measuring metabolites are seen for androgens and adrenal hormones, as will be discussed in the following case study. Finally, 24-hour urine makes possible the measurement of hormones which are secreted mainly at night and those with very short half-lives, such as melatonin, oxytocin, and growth hormone.
The aim of developing dry urine hormone panels in recent years has been to combine the circadian adrenal patterns available in a four-point salivary cortisol with the extensive metabolite data obtainable in urine. Our medical laboratory is associated with a busy clinical practice of integrative practitioners, and have accumulated over 40 years of clinical and research data on the efficacy and utility of 24-hour urine hormone results. It was this understanding and experience that we drew upon in the development and validation of our dry urine hormone panel. What follows is a comparative analysis of a 24-Hour Urine Hormone Panel and a Four-Point Dry Urine Hormone Panel collected from the same patient on the same day.
The Use of GS/MS and LC/MS/MS in Urine Hormone Assays
Some background on the methodology used for urine hormone testing may be helpful. Historically known as a reference method, gas chromatography/mass spectrometry (GC/MS) has been a time-proven analytical technique for urinary steroid hormone profiling in industry and academia since the 1960s. A single run is able to capture all steroids excreted in a given urine sample. A prime example of its merits is the characterization of inborn errors of metabolism of which have almost exclusively been carried out by GC/MS. Liquid chromatography/tandem mass spectrometry (LC/MS/MS) is another exceptional separation technique to quantitate hormones and their metabolites from a urine matrix. Compared to LC/MS/MS, GC/MS is known for inherently better chromatographic resolution. Yet, LC/MS/MS has seen exceptional growth during the last ten to fifteen years particularly for its high throughput and high analytical specificity and sensitivity.(4,5) The performance characteristics of these two techniques for steroid analysis, both have unique advantages and complement each other. Combined, these two techniques offer a more comprehensive testing platform than one alone. Both techniques surpass immunoassays in clinical utility. Problems inherent to immunoassays; cross-reactivity impacting specificity, large inter-assay variability, and limits in measuring low concentrations of hormones, make GC/MS and LC/MS/MS superior in terms of specificity, limits of quantification and reliability.
24-Hour And Dry Urine Collection
A 24-hour urine collection involves collecting all urine output for an entire 24-hour period. The patient is provided with a urine collection cup and a three-liter, amber-colored collection jug. If the patient begins collection at 7am Monday, for example, it would be completed at exactly 7am Tuesday. If any specimen is missed or spilled at any point during the collection time, the patient should discard all urine, and recollect. Most patients arrange to collect on a weekend or other day when they will be home most of the day. While some patients find 24-hour collection somewhat cumbersome, this method still provides for the most comprehensive steroid hormone profiling on the market today. It includes the measurement of peptide hormones not currently available through dry urine; free T3 and T4, melatonin, oxytocin and growth hormone.
Dry urine testing is touted for its ease of specimen collection and reporting of the cortisol/cortisone circadian rhythm. It involves collecting a urine sample at four different times throughout the day on a filter paper card; the first morning, two to three hours afterward, before dinner and before bedtime. Urine is collected into a cup, the collection card is submerged into the urine to allow complete saturation and the card is then taped to the edge of a countertop for air-drying. Many patients prefer this method to having to collect all urine for 24 hours. Others find that the precise timing required for the dry urine method to be less convenient than a 24-hour collection. The dry urine method includes all the steroid hormone metabolites included in a 24-hour collection, except aldosterone. It does not include growth hormone (hGH), thyroid hormones (FT3, FT4), oxytocin, or melatonin. The melatonin metabolite, 6-sulphatoxymelatonin, is available in the Dry Urine Profile. The four, timed collections allow four-point cortisol and cortisone curves to be plotted in the Dry Urine Hormone Profile, but not the 24-Hour Urine Hormone Profile. The Dry Urine Hormone Profile costs less than the more comprehensive 24-Hour Urine Hormone Profile.
Comparing 24-hour Urine to Dry Urine
Values on the 24-hour Urine Hormone Profile cannot be compared directly with values on the Dry Urine Hormone Profile. Although analytical methods are the same (GC/MS/MS), different collection methods result in differing measurements and reference ranges. The use of filter paper for obtaining the sample has been shown to be highly accurate, yielding comparable levels of metabolites as using liquid urine directly. There are no matrix effects of the filter paper that interfere with recovery. For purposes of this comparison, we will be using the medians of the reference ranges (RRM). Clinical observation and research have helped to delineate optimal levels of many hormones in the 24-Hour Urine Profile, and to understand how those relate to the RRM. It is not expected that Dry Urine values relative to RR will exactly mirror 24-Hour Urine values. Because hormones fluctuate hour by hour, collection at any one point in time can reflect peak values, baseline values, or something in between. Variability is greatest for those hormones that have the shortest half-life. 24-hour collection averages out these differences for the entire collection period, whereas four-point collection for Dry Urine includes only the values at the four collection points. Dry Urine percentages can be expected to vary 10-15% from 24-hour percentages, and sometimes more, but the overall trend should be the same. Either method provides a sound basis for treatment decisions.
Case Study: Mary Smith
Mary is a 47-year-old, pre-menopausal woman whose physician recommended a urine hormone profile. She was offered a free test in exchange for agreeing to collect for both the 24-hour collection method and the dry-urine method during the same 24-hour period. Mary collected on day 21 of her menstrual cycle (as recommended in collection instructions) and her total volume for the 24-hour collection was 2800mL, which is a normal volume for a well-hydrated woman. Urine collections at 6:25AM, 8:45 AM, 4:25PM, and 10:15PM were dipped for the Dry Urine portion of the test.
Mary is 5’0” tall, weighs 108 pounds and has a small frame. She works out regularly at the gym, is very conscientious about her health, tries to eat well, and looks young for her age. Mary has a very positive, upbeat attitude, but has been under considerable stress in the past few years. Her sister passed away two years ago from ovarian cancer. Until recently she had worked for more than a decade in a difficult and stressful work environment that left her feeling unappreciated and fearful of her job security, although she was a model employee. Recently, she accepted a position with another company where she feels more professionally fulfilled and respected. She has met a man whose company she enjoys and is feeling positive about the future. She is generally healthy but, because of her small stature, she has an increased risk of osteopenia and osteoporosis.
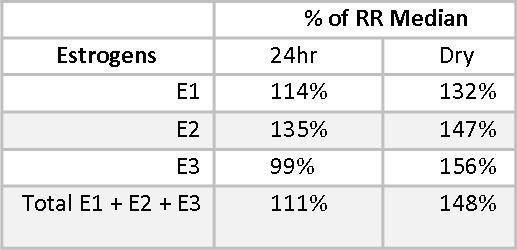
Estriol (E3) is considered a protective estrogen because it binds to Estrogen Receptor β (ERβ), which increases differentiation of cells and decreases proliferation of cells.(6-8) E1 and E2 have important benefits, but also have potentially carcinogenic metabolites.(9) The Estrogen Quotient (EQ)7 is calculated by dividing E3 by the total of E1+E2, and should ideally be >1.5. Some practitioners prefer to see it >2.0. Mary’s EQ is lower than optimal. We do not expect the EQ on the 24-hr urine profile and the Dry Urine profile to be identical, but we do expect them to be consistent in what they are telling us, and both EQs are significantly lower than 1.5. Supplemental Iodine may be helpful in improving Mary’s EQ.

Phase I and Phase II estrogen metabolites also give insight into the health of Mary’s estrogen metabolism. The Phase I metabolite, 2-Hydroxyestrone (2OHE1), is considered a protective estrogen;(10,11) 4-Hydroxyestrone (4-OHE1) and to a lesser extent, 16α-Hydroxyestrone (16αOHE1), are considered carcinogenic. We don’t want to see these estrogen metabolites too high, even when staying within the reference range. 16αOHE1 serves an important role in maintaining bone density, and when it is too low, women may be at increased risk for osteopenia and osteoporosis.(12,13) Mary has healthy levels of 2OHE1, at 91% and 92.4% of the reference range median (RRM) for the 24-hour and the Dry Urine samples, respectively.
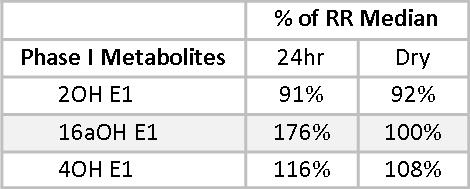
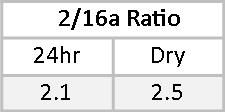
16αOHE1 percentages of the RRM are less closely matched, primarily due to a wider RR in the dry urine. This is adjusted for by a leftward shift in the optimal range as represented on the dry urine report. In both cases, the 16αOHE1 falls outside the optimal range. The ratio of 2OHE1/16αOHE1 represents the balance between protective and carcinogenic Phase I metabolites.(14-18) A 2/16α ratio of between two and four is considered healthy. Less than two suggests an increased risk of developing estrogen-related cancer. More than four may be indicative of risk for osteopenia, especially when the actual 16αOHE1 value is very low. Mary’s 2/16α ratios are 2.1 and 2.5 on the two tests. While these fall within the optimal range, given Mary’s family history and that her actual 16αOHE1 values are above optimal, some DIM or I3C might be helpful to reduce 16αOHE1 levels and increase the 2/16α ratio.
Mary’s levels of 4OHE1 are at 116% and 108% of RRM. These levels are consistent and are somewhat concerning given her family history of ovarian cancer. 4OHE1 levels may be decreased by providing supplemental magnesium and support for methylation.(19)
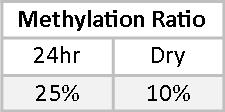
Phase II metabolites allow us a peek into the balance between Phase I and Phase II detoxification. 2-Methoxyestrone (2MeoE1) is metabolized directly from the Phase I metabolite 2OHE1. Although the optimal ratio of 2MeoE1 to 2OHE1 is not known, we believe that this ratio should be at least 0.50, or 50%. Mary’s methylation ratio is significantly less than 50% on both tests.
2-Methoxyestradiol (2MeoE2) is the most protective of the estrogen metabolites and also the one produced in the smallest amounts.(20-23) Mary’s 2MeoE2 falls well below the median in both tests. We are unaware of any specific treatments that will increase 2MeoE2 levels at this time. Supplemental 2MeoE2 is available through some compounding pharmacies.
In comparing the 24-hour Urine and Dry Urine reports, we see that treatment recommendations would be the same for both reports. Differences in values and percentage of RRM between the reports are reasonable and explainable variations between the two methods.
Progesterone
Progesterone levels are much more stable than estrogen. Estrogens have a relatively short half-life, and exogenous estrogen can be expected to have effectively vanished from the body within 24 hours of using it. Progesterone, with its much longer half-life, is stable in serum after five days of daily use and does not return to baseline for two to three weeks after being discontinued.(24) This shows up quite clearly in the comparison of pregnanediol levels in 24-hour Urine and Dry Urine profiles. The progesterone metabolite, pregnanediol, is well established in the literature as a reliable marker for Progesterone.(25) Progesterone itself is not measured in urine because its structure does not lend itself to urine excretion. However, Mary’s pregnanediol levels, as a percentage of RRM are 69% and 68.8%, demonstrating a remarkable consistency between the two methods.
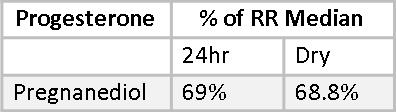
This consistency was also observed in the reports of other patients who did side-by-side testing. For example, in one young woman with PCOS percentages of RRM for pregnanediol were 19% by both methods.
For Mary, these levels represent robust progesterone production. Progesterone levels decline earlier in a woman’s reproductive life than do estrogens. It is not at all unusual to see pregnanediol values for a woman in her mid-late forties well down below the luteal range.
Androgens
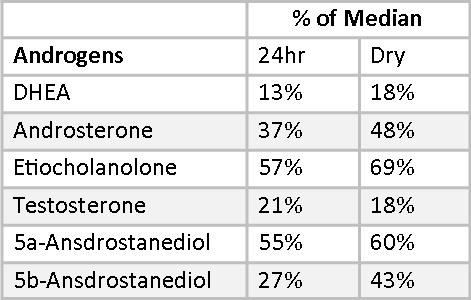
Mary’s androgens are uniformly at the low end of the reference ranges, with testosterone falling below normal on both 24-hr and Dry Urine samples. DHT is also low in the Dry Urine sample. DHT is not measured in 24-hr urine profiles. In addition to measuring DHEA, Testosterone, and DHT, it is important to measure androgen metabolites. Androgen metabolites are sometimes a better measure of androgen activity than the primary androgens. In Mary’s case, the androgen metabolites confirm the low testosterone and DHT numbers. The Dry Urine percentages of RRM very closely tracks the 24-hr Urine percentages, differing by between 2-12% for all androgens except for 5,-Androstanediol, which differs by 16%, still an acceptable variation. Most important, both the 24-hr Urine Profile and the Dry Urine Profile point to overall low androgen production, which may be ameliorated with botanical therapies or supplemental hormones.
Glucocorticoids and Mineralocorticoids
Measuring cortisone and cortisol metabolites allows a fuller assessment of adrenal function and reserves than measuring cortisol alone (or cortisol and DHEA). Both the 24-hour Urine Hormone Profile and the Dry Urine Profile measure both cortisone and cortisol, as well as a number of glucocorticoid and mineralocorticoid metabolites.
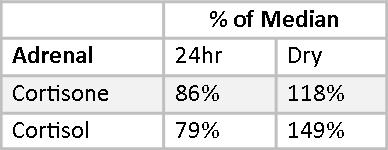
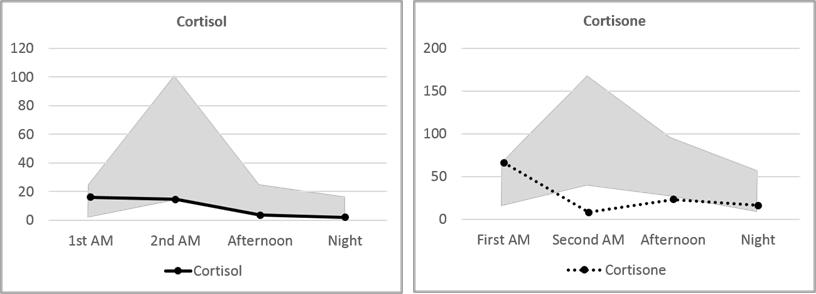
Mary’s Cortisol and Cortisone fall slightly below the median on the 24-hour Urine Panel and somewhat above the median on the Dry Urine Hormone Panel. The variance between the two is larger than for most other hormones, and this is expected because the relationship between cortisol and cortisone is quite dynamic. Over the period of a day, cortisol converts to cortisone and cortisone back to cortisol continuously. A 24-hour collection averages out these shifts; a four-point collection will necessarily show the shifts more distinctly.
More clinically relevant are the three metabolites, Tetrahydrocortisone (THE), allo-Tetrahydrocortisol (5-THF), and Tetrahydrocortisol (THF). These three, together, account for about half of daily cortisol production.(26-28) The total of these, reported as Adrenal Reserve, should be at 100% of RRM or higher. Mary’s Adrenal Reserve is remarkably consistent between the testing methods, adding up to 53% and 58% of the median – a little over half of what would be optimal. This accords well with Mary’s reported symptoms of morning and evening fatigue.
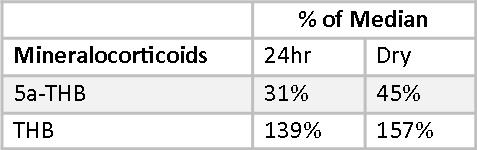
Mary’s mineralocorticoid, allo-tetrahydrocorticosterone (5-THB), is well below RRM in both reports. Her Tetrahydrocorticosterone (THB) is well above RRT in both reports. This disparity in metabolite levels are reflective of very low 5-reductase activity, and are consistent between the two methods. Otherwise, her mineralocorticoids appear normal.

Four-Point Cortisol and Cortisone
The circadian cortisol pattern measured in four salivary samples has been used for many years to evaluate the normalcy of daily cortisol production in an individual. In general, cortisol is expected to be higher in the morning, shortly after awakening, and fall throughout the day with the lowest levels occurring at night. Low morning cortisol or high evening cortisol are deviations from the normal pattern that can explain clinical symptoms and are often amenable to therapeutic intervention.
In urine testing of hormones, both cortisol and cortisone are measured. This gives a more comprehensive picture of hormonal balance because, as mentioned above, there is a dynamic interchange of cortisol and cortisone throughout the day. Perhaps as important as measuring both cortisone and cortisol is measuring both the free and the conjugated fraction of each hormone. Free cortisone and cortisol are bioavailable cortisone and cortisol. Conjugated cortisone and cortisol represent free hormone that has been conjugated for excretion. The remaining free portion is a very tiny fraction of the total bioavailable hormone. Thus, measuring conjugated and free hormone captures the entire range of bioavailable hormone.
In saliva, the cortisol value represents circulating cortisol from about an hour previous to the collection. A typical salivary cortisol curve starts high in the early morning and declines throughout the day. In urine, cortisol and cortisone values represent circulating cortisol from about five hours prior to collection. Thus, normal urine cortisol and cortisone curves start low and show a distinct peak at the second-morning collection, before descending for the rest of the day. Although Mary’s cortisol and cortisone values are in general below or near the bottom of the normal range, her first-morning cortisol and cortisone are higher than any other point of collection. Since this represents hormone levels five or more hours previously, it is indicative of higher nighttime cortisol levels followed by a daytime drop: the opposite of a healthy cortisol curve.
Melatonin and 6-Sulfatoxymelatonin
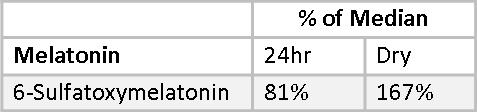
Melatonin cannot be measured directly using the Dry Urine method. Melatonin is light sensitive and breaks down on the filter paper used to collect the urine. Instead, 6-sulfatoxymelatonin is measured. Less than 1% of endogenous melatonin is excreted in urine in the unmetabolized form. The concentration of its major metabolite, 6-sulfatoxymeltonin is two to three orders of magnitude higher and, therefore, widely used as a stable marker of endogenous melatonin in the blood. However, with oral supplementation, estimation of urinary melatonin may serve as a more reliable projection of blood melatonin levels over its metabolite due to variable first pass effects found among individuals.(29) In a Dry-Urine collection, 6-sulfatoxymelatonin is measured only in the first-morning urine sample, which reflects melatonin production the previous evening and night. This is not expected to correlate with a 24-hour Urine collection, which will be more dilute. This is demonstrated in Mary’s 6-sulfatoxymelatonin values, which fall at 85% of the median for the 24-hour sample and are 167% of median for the Dry Urine sample. Melatonin can be measured directly in the 24-hour sample which is protected from light degradation by an amber container. Mary’s 24-hour Melatonin value is 147% of the median.
Urine Hormones Measured Only in 24-Hour Collections
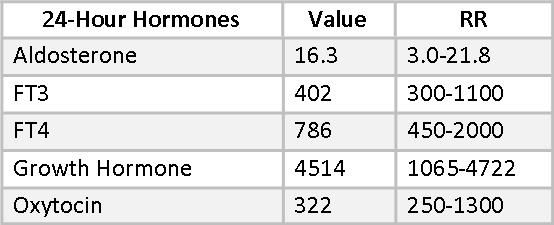
Besides Melatonin, there are several hormones included in Mary’s 24-hour urine hormone collection that are not available in the dry urine method at this time. They include free T3, free T4, aldosterone, growth hormone, and oxytocin.
Low aldosterone is sometimes associated with age-related hearing loss(30-32) and supplemental aldosterone has been helpful in some cases in restoring hearing acuity. Mary’s aldosterone is at a healthy level and she reports no hearing deficits.
24-hour urine free T4 and, especially, free T3 can be sensitive indicators of sub-clinical thyroid hypofunction.(33) The relationship between FT4 and FT3 is also of interest. Significantly more FT4 than FT3 suggests that 5’-Deiodinase is being inhibited and the alternative 5-Deiodinase pathway has been activated, leading to higher reverse T3 (RT3). This can be a result of stress, high cortisol or heavy metal toxicity. Mary’s FT4 value of 786 is near the optimal range, but her FT3 , at only 402, is well below optimal. The dual stresses in Mary’s life of a hostile work environment and the illness and death of her sister are certainly enough to have impacted this pathway. Additional selenium may be helpful here, as may the change in her job circumstances.
Mary’s growth hormone (GH) is a healthy 4514, well into the upper end of the RR. This is a “healthy aging” reference range, derived from 18-35-year-olds. Robust GH is often seen in people who look younger than their chronological age.
Oxytocin is a hormone with a growing list of promising applications. (See Beyond Sex, Birth, and Breastfeeding: Promising Clinical Uses for Oxytocin. Townsend Letter, Feb/March 2016)). Mary’s oxytocin is in the lower part of the reference range. She is not reporting any symptoms associated with conditions that might benefit from oxytocin supplementation, at this time. Oxytocin may be helpful in maintaining bone density, so this would be something to consider if future testing revealed osteopenia.
Conclusion
The 24-Hour Urine Hormone Profile and the Dry Urine Hormone Profile are equally useful in assessing levels of sex hormones and adrenal function. Because of the range of metabolites available in urine, these profiles provide a more reliable and clinically useful understanding of a patient’s hormonal status than either saliva or serum. The 24-Hour Urine Hormone Profile has some additional benefits in the ability to measure growth hormone, oxytocin and thyroid hormones. Additional benefits of the Dry Urine Hormone Profile include a greater understanding of circadian cortisol and cortisone patterns and a lower cost. Both panels have been shown to be a useful tool in monitoring bioidentical hormone replacement therapy for safety and efficacy.
References
1. Eliassen AH, Ziegler RG, Rosner B, et al. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009 Nov;18(11):2860-8. Epub 2009 Oct 20. PMID: 19843676
2. Falk RT, Xu X, Keefer L, et al. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008 Dec;17(12):3411-8. PMID: 19064556
3. Faupel-Badger JM, Fuhrman BJ, Xu X, et al. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010 Jan;19(1):292-300. PMID: 20056650
4. Krone N, Hughes BA, Lavery GG, et al. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010 Aug; 121(3-5):496-504. PMID: 20417277
5. Grebe SKG, Ravinder JS. LC-MS/MS in the clinical laboratory – where to from here? Clin Biochem Rev. 2011 Feb; 32(1):5-31. PMID: 21451775
6. Maehle BO, et al. Estrogen receptor beta – an independent prognostic marker in estrogen receptor alpha and progesterone receptor-positive breast cancer? APMIS. 2009. Sep;117(9):644-50.
7. Imanov O. et al. Estrogen receptor in health and disease. Biol Reproduct. 2005. 73(5):866-71.
8. Zhu, Han et al. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology, 2006 147:4132-4150
9. Lemon HM et al. Reduced estriol excretion in patients with breast cancer prior to endocrine therapy. JAMA. 1966 June 27;196(13):1128-36.
10. Telang NT et al. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992; 84 (8): 634-638.
11. Jernstrom H, et al. Predictor pf the plasma ratio pf 2-hydroxyestrone to 16(alpha)-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24(5):991-1005.
12. Lim SK, Won YJ, Lee JH, e al, Altered hydroxylation of estrogen in patient with postmenopausal osteopenia. J Clin Endocrinol Metab. 1997; 82(4):1001-6.
13. Lotinun S. et al. Tissue selective effects of continuous release of 2-hydroxyestrone and 16alpha-hydroxyestrone on bone, uterus and mammary gland in ovariectomized growing rats. J Endocrinol. 2001. 170(1):165-74.
14. Kabat GC, O’Leary ES, Gammon MD et al. Estrogen metabolism and breast cancer. Epidemiology 2006; 17 (1): 80-8.
15. Fuhrman BJ. et al. Estrogen metabolism and the risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012. Feb 22;104(4):326-39.
16. Falk RT. et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2013. Apr 22;15(2):R34.
17. Dalessandri KM. et al. Pilot study: Effect of 3,3’-diindolymethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutrition and Cancer. 2004. 50(2):161-7.
18. Anderson et al. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012 Aug;23(8):853-9.
19. http://www.genecards.org/cgi-bin/carddisp.pl?gene=COMT&search=COMT
20. Lakhani NJ et al. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003. Feb;23(2):165-72.
21. Kuo et al. 2-methoxyestradiol induces mitotic arrest, apoptosis, and synergistic cytotoxicity with arsenic trioxide in human urothelial carcinoma cells. PLoS One. 2013 Aug 13;8(8):e68703.
22. Wang G et al. Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth and inducing apoptosis in human prostate cancer cells. Oncol Rep. 2013 Jul;30(1):357-63
23. Choi HJ et al. Critical role of cyclin B1/Cdc2 up-regulation in the induction of mitotic prometaphase arrest in human breast cancer cells treated with 2-methoxyestradiol. Biochim Biophys Acta. 2012 Aug;1823(8):1306-15.
24. https://www.drugbank.ca/drugs/DB00396
25. Munro CJ et al. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem. 1991 Jun;37(6):838-44.
26. Burtis CA et al. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia, PA W.B. Saunders Company; 1999.
27. Tomlinson J et al. 11β-hydroxysteroid dehydrogenase type 1: A tissue specific regulator of glucocorticoid response. Endocrine Reviews. 2004. 25(5);831-866.
28. Jerjes WK et al. Urinary cortisol and cortisol metabolite excretion in chronic fatigue syndrome. Psychosom. Med. 2006;68(4):578–82.
29. Kovacs J, Brodner W, Kirchlechner V, et al. Measurement of urinary melatonin; a useful tool for monitoring serum melatonin after its oral administration. J Clin Endocrinol Metab. 2000 Feb;85(2):666-70. PMID: 10690873
30. Trune DR, Kempton JB, Kessi M. Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MpJ-Fas1pr autoimmune mice. Laryngoscope. 2000;110(11):1902-1906..
31. Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: a possible protective hormone against presbycusis. Hear Res. 2005;209(1-2):10-18.
32. Halonen J, Hinton AS, Frisina RD, Ding B, Zhu X, Walton JP. Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear Res. 2016;336:63-71.
33. Baisier WV. et al. Thyroid insufficiency. Is TSH measurement the only diagnostic tool? J Nutr Environ Med. 2000;(10):105-113
